Aug 31, 2025
TUNEL staining to detect apoptotic cells in formalin-fixed, paraffin-embedded (FFPE) pig tissues
Forked from a private protocol
- Jayne Wiarda1,
- Colin Stoy1
- 1National Animal Disease Center, ARS, USDA
- Jayne Wiarda

Protocol Citation: Jayne Wiarda, Colin Stoy 2025. TUNEL staining to detect apoptotic cells in formalin-fixed, paraffin-embedded (FFPE) pig tissues. protocols.io https://dx.doi.org/10.17504/protocols.io.5jyl88dwrl2w/v1
License: This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Protocol status: Working
We use this protocol and it's working
Created: August 31, 2025
Last Modified: August 31, 2025
Protocol Integer ID: 226104
Keywords: NP103, apoptotic cells in formalin, indicative of cellular apoptosi, cellular apoptosi, apoptotic cell, porcine tissue, pig tissue, situ identification of dna fragmentation, dna fragmentation, ffpe
Funders Acknowledgements:
USDA-ARS
Grant ID: CRIS #5030-32000-230-000-D
Disclaimer
All opinions expressed in this paper are the authors’ and do not necessarily reflect the policies and views of USDA or ARS. Mention of trade names or products is for information purposes only and does not imply endorsement by the USDA. USDA is an equal opportunity employer and provider.
Abstract
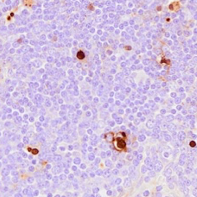
A protocol for in situ identification of DNA fragmentation indicative of cellular apoptosis in formalin-fixed, paraffin-embedded (FFPE) porcine tissues.
Attachments
Guidelines
Assay Controls
· Negative control
o This slide receives only diluent in place of DNase1 and TdT Enzyme. All other reagents are still applied to the slide.
· Positive control
o This is the only slide that receives diluted DNase1 to induce apoptosis before detection by TdT Enzyme. All other reagents are still applied to the slide.
Assay Variations
· Parameters may need to be further optimized for different experiments, tissues, targets, or species.
Materials
Equipment
· Pipettes/pipette tips
· Drying oven (able to reach & hold 60℃)
· Fume hood
· Slide staining tray (e.g. Simport M920-2)
· HybEZ II Hybridization System with ACD EZ-Batch Slide System (Advanced Cell Diagnostics [ACD] 321710/321720)
o HybEZ oven (ACD 321710/321720)
o Humidity control tray (ACD 310012)
o HybEZ Humidifying Paper (ACD 310025)
o EZ-Batch Wash Tray (ACD 321717)
o EZ-Batch Slide Holder (ACD 321716)
· Tissue-Tek Vertical 24 slide rack (American Master Tech Scientific LWS2124)
· Tissue-Tek Staining Dishes (American Master Tech Scientific LWS20WH)
· Tissue-Tek Clearing Agent Dishes, xylene resistant (American Master Tech Scientific LWS20GR)
· Brightfield microscope and/or slide imaging platform
Reagents/Supplies
· Distilled water (obtained in-house)
· PBS, pH 7.2 (made in-house )
· 0.05% PBS-Tween (PBS-T), pH 7.35 (made in-house)
· Xylenes (Macron Fine Chemicals 8668-16)
· 100% ethanol (Pharmco 111000200)
o Dilute with distilled water to make 95%, 85%, and 70% concentrations
· Pro-Par Clearant (Anatech 510)
· Fixative
o 10% NBF (Cancer Diagnostics, Inc. 111) or 4% PFA (Electron Microscopy Sciences 15713)
· ImmEdge Hydrophobic Barrier Pen (Vector H-4000)
· BLOXALL Endogenous Blocking Solution (Vector SP-6000)
· TUNEL In Situ Apoptosis Kit (HRP-DAB Method) (Elabscience, E-CK-A331)
o TdT Equilibration Buffer (E-CK-A32A)
o TdT Enzyme (E-CK-A32B)
o Proteinase K (100X) (E-CK-A32C)
o Streptavidin-HRP (E-CK-A331D)
o Biotin-dUTP (E-CK-A331E)
o DAB Concentrate(20X) (E-CK-A331F)
o DAB Dilution Buffer (E-CK-A331G)
o DNase I (20 U/μL) (E-CK-A32E)
o DNase I Buffer (10X) (E-CK-A32F)
· Gill’s Hematoxylin I (American Master Tech Scientific HXGHE1LT)
· Refrax Mounting Medium (Anatech 711)
· #1 thickness cover glass (e.g. Fisherbrand 12-545-F)
Troubleshooting
Safety warnings
***For all reagents, refer to MSDS to determine appropriate precautions, personal protective equipment (PPE), and disposal methods before use***
Before start
Starting specimens
Starting samples = FFPE tissues cut to 4 micron thickness and adhered to positively-charged microscopy slides (e.g. SuperFrost Plus Slides; Fisher Scientific 12-550-15). It is crucial that tissues are adequately fixed to prevent tissue degradation. Tissues no thicker than 0.5 centimeters should be freshly harvested and placed into 10% neutral-buffered formalin (NBF) or 4% paraformaldehyde (PFA) at a ratio of at least 20 volumes fixative per one volume tissue. Fix tissues between 16-30 hours at room temperature (RT), followed by immediate transfer to 70% ethanol and processing into FFPE tissue blocks. Fixation times should be optimized for individual tissues and experiments.
Baking
20m
Before starting the assay:
• Preheat a dry oven to 60℃
• Load slides for assay into vertical slide rack
• Bake slides 20 min 60℃00:20:00
20m
While slides bake:
o Prepare 0.05% PBS-T
Immediately before deparaffinizing:
o Add ~200 mL xylenes to each of three clearing agent dishes in a fume hood
o Add ~200 mL 100% ethanol to each of two staining dishes in a fume hood
o Add ~200 mL 95% ethanol to a staining dish in a fume hood
o Add ~200 mL 85% ethanol to a staining dish in a fume hood
o Add ~200 mL 70% ethanol to a staining dish in a fume hood
o Add ~200 mL distilled water to a staining dish in a fume hood
o Add ~200 mL PBS-T to a staining dish
Deparaffinizing & Rehydrating
20m
o Submerge slide rack in fresh xylenes 5 min RT 00:20:00
o Submerge slide rack in fresh xylenes 5 min RT
o Submerge slide rack in fresh xylenes 5 min RT
o Submerge slides rack in fresh 100% ethanol 1 min RT
o Submerge slides rack in fresh 100% ethanol 1 min RT
o Submerge slides rack in fresh 95% ethanol 1 min RT
o Submerge slides rack in fresh 85% ethanol 1 min RT
o Submerge slides rack in fresh 70% ethanol 1 min RT
o Submerge slides rack in fresh distilled water 3 min RT
o Submerge slides rack in fresh PBS-T for transport
20m
While slides deparaffinize/rehydrate:
o Turn off dry oven
o Prepare humidified slide staining tray by adding water to bottom & placing lid on top
Hydrophobic Barrier
20m
• Apply hydrophobic barrier around each tissue 00:20:00
o One by one, unload slides from vertical rack submerged in PBS-T. Dry off only the area around the tissue where a barrier will be drawn with a hydrophobic barrier pen. Keep tissue area wet the whole time. Draw barrier and place slides into the EZ-Batch slide holder placed inside the slide staining tray. Using a pipette, apply a small amount of PBS-T within the barrier (just enough to keep the tissue wet while drawing barriers on remaining slides). Slides will remain locked in the EZ-Batch slide holder throughout the protocol until being transferred back to a vertical rack for counterstaining.
• Leave slide holder in slide staining tray
20m
Immediately before Proteinase Digestion:
o Prepare 1X Proteinase K by diluting 1 part 100X Proteinase K in 99 parts PBS. Total volume to use is dependent on tissue sizes. Make sure to mix reagents before pipetting.
Proteinase K Digestion
25m
• Decant slide holder and again place flat in slide staining tray 00:25:00
• Incubate with 1X Proteinase K 20 min at 37C
o Apply completely cover tissues; let incubate in humidifying tray placed within preheated HybEZ oven
o Manufacturer recommends optimization of this step
• Remove slide holder from HybEZ oven, decant, and transfer to wash trays for PBS-T washes
• Submerge slide holder in fresh PBS-T 2 min RT
• Submerge slide holder in fresh PBS-T 2 min RT
25m
While slides incubate with proteinase:
o Discard deparaffinizing & rehydrating reagents
o Add ~200 mL PBS-T to each of two staining dishes
Tissue Quenching
15m
• Decant slide holder and again place flat in slide staining tray 00:15:00
• Incubate with BLOXALL Endogenous Blocking Solution 10 min RT
o Apply to completely cover tissues; let incubate in slide staining tray with lid closed
• Decant slide holder and transfer to wash trays for PBS-T washes
• Submerge slide holder in fresh PBS-T 2 min RT
• Submerge slide holder in fresh PBS-T 2 min RT
o Hold target samples in PBS-T until positive and negative slide are prepared
15m
While slides incubate with enzyme block:
o Discard proteinase reagents
o Add ~200 mL PBS-T to each of two wash trays
o Prepare 1X DNase Buffer by diluting 1 part 10X DNase1 Buffer in 9 parts distilled water. Prepare enough volume for only control slides, and total volume to use is dependent on tissue sizes.Make sure to mix reagents before pipetting.
DNase (control slides only)
30m
• Remove positive and negative control slides from slide holder, decant, and place on flat surface
• Incubate positive and negative control slides with 1X DNase Buffer 5 min RT
• Decant slides and place in humidifying tray taken from preheated HybEZ oven
• Incubate with DNase Working Solution to positive control only and 1X DNase Buffer to negative control only 20 min 37C
o Apply to completely cover tissues; let incubate in humidifying tray placed within preheated HybEZ oven
o Manufacturer recommends optimization of this step
• Remove slides from HybEZ oven, decant, and transfer back to wash tray still containing non-control slides for PBS-T washes
• Submerge slide holder in fresh PBS-T 2 min RT
• Submerge slide holder in fresh PBS-T 2 min RT
30m
While slides incubate with DNase Buffer:
o Prepare DNase Working Solution by diluting 1 part 100X DNase1 in 99 parts 1X DNase 1 Buffer, preparing enough volume to cover tissue on the positive control slide only. Total volume to use is dependent on tissue sizes. Make sure to mix reagents before pipetting.
While slides incubate with DNase:
o Discard tissue quenching reagents
o Bring TdT Equilibration Buffer to RT and ensure any crystallization is fully dissolved before use
o Prepare TdT Enzyme Working Solution by diluting 1 part Biotin-dUTP and 1 part TdT Enzyme in 8 parts TdT Equilibration Buffer. TdT Enzyme should be kept at -20C and only be taken to RT as long as needed to add to TdT Enzyme Working Solution. Total volume to use is dependent on tissue sizes. Make sure to mix reagents before pipetting, and only mix enzyme and working selection via pipetting; do not vortex.
TdT Labeling
1h 30m
• Decant slide holder and place flat in humidifying tray
• Incubate with TdT Equilibration Buffer 20 min 37C
o Apply to completely cover tissues; let incubate in humidifying tray placed within preheated HybEZ oven
o Manufacturer recommends optimization of this step
• Remove slide holder from HybEZ oven, decant, and place in humidifying tray
• Incubate with TdT Enzyme Working Solution 60 min 37C
o Apply to completely cover tissues; let incubate in humidifying tray placed within preheated HybEZ oven
• Remove slide holder from HybEZ oven, decant, and transfer to wash trays for buffer washes
• Submerge slide holder in fresh PBS-T 2 min RT
• Submerge slide holder in fresh PBS-T 2 min RT
1h 30m
While slides incubate with TdT Equilibration Buffer:
o Prepare TdT Enzyme Working Solution by diluting 1 part Biotin-dUTP and 1 part TdT Enzyme in 8 parts TdT Equilibration Buffer. TdT Enzyme should be kept at -20C and only be taken to RT as long as needed to add to TdT Enzyme Working Solution. Total volume to use is dependent on tissue sizes. Make sure to mix reagents before pipetting, and only mix enzyme and working solution via pipette; do not vortex.
While slides incubate with TdT Enzyme Working Solution:
o Discard DNase reagents
o Prepare Streptavidin Working Solution by diluting 1 part 100X Streptavidin in 99 parts PBS. Total volume to use is dependent on tissue sizes. Make sure to mix reagents before pipetting.
Streptavidin Development
35m
• Decant slide holder and again place flat in humidifying tray
• Incubate with Streptavidin Working Solution 30 min 37C
o Apply to completely cover tissues; let incubate in humidifying tray placed within preheated HybEZ oven
• Remove slide holder from HybEZ oven, decant, and transfer to wash trays for buffer washes
• Submerge slide holder in fresh PBS-T 2 min RT
• Submerge slide holder in fresh PBS-T 2 min RT
o Washing time can be extended to reduce residual HRP background staining
35m
While slides incubate with Streptadivin:
o o Discard TdT Labeling Reagents
o Prepare diluted DAB chromogen by diluting 1 part 20X DAB in 19 parts DAB Dilution Buffer. Total volume to use is dependent on tissue sizes. Make sure to mix reagents before pipetting. Store in the dark due to light sensitivity.
Chromogenic Detection (DAB)
10m
• Decant slide holder and again place flat in slide staining tray 00:10:00
• Incubate with diluted DAB chromogen 3 min RT
o Apply to completely cover tissues; let incubate in slide staining tray with lid closed
o Time of DAB incubation can be adjusted according to reaction rate
• Decant slide holder and transfer to wash trays for PBS-T washes
• Submerge slide holder in fresh PBS-T 2 min RT
• Submerge slide holder in fresh PBS-T 2 min RT
10m
While slides incubate with chromogen:
o Discard remaining secondary antibody reagents
o Add ~200 mL PBS-T to each of two wash trays
o Add ~200 mL hematoxylin to one staining dish
o Add ~200 mL distilled water to each of three staining dishes
Counterstaining
3m
• Transfer slides to vertical slide rack00:03:00
o Do quickly to avoid drying out slides or alternatively place vertical slide rack in a staining dish containing PBS-T and then transfer slides
• Submerge slide rack in hematoxylin 20 sec RT
• Submerge slide rack in fresh distilled water, dunking 3-5 times
• Submerge slide rack in fresh distilled water, dunking 3-5 times
• Submerge slide rack in fresh distilled water, dunking 3-5 times
o Water should no longer appear purple in the third water dish used
3m
Mounting
• Submerge slide rack in fresh 95% ethanol 1 min RT
• Submerge slide rack in fresh 100% ethanol 1 min RT
• Submerge slide rack in fresh 100% ethanol 1 min RT
• Submerge slide rack in fresh 100% ethanol 1 min RT
• Submerge slide rack in fresh Pro-Par 5 min RT
• Submerge slide rack in fresh Pro-Par 5 min RT
• Submerge slide rack in fresh Pro-Par 5 min RT
• Mount slides by adding 2-4 drops of mounting media to each slide, followed by application of a cover glass. Remove bubbles from tissue by applying pressure to cover glass
• Place slides flat in a dry, dark space to air dry at RT overnight
• Assess staining with a brightfield microscope
While slides are air drying:
• Discard chromogen detection, counterstaining, and slide mounting reagents
Protocol references
· Staining protocol was developed by Dr. Jayne Wiarda and Colin Stoy
· Staining protocol was optimized and executed by Colin Stoy
· We thank Adrienne Shircliff for slide sectioning and imaging

