Nov 22, 2020
Sample preparation protocol for cross-sectional microscopy of hair fibers
- Tina Lasisi1
- 1University of Michigan

Protocol Citation: Tina Lasisi 2020. Sample preparation protocol for cross-sectional microscopy of hair fibers. protocols.io https://dx.doi.org/10.17504/protocols.io.bbwcipaw
License: This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Protocol status: Working
We use this protocol and it's working
Created: January 29, 2020
Last Modified: November 22, 2020
Protocol Integer ID: 32420
Keywords: hair morphology, cross-sectional morphology, microscopy, hair fiber shape, embedding, hair fibers this protocol, hair fiber, sample preparation protocol, preparation,
Abstract
This protocol describes how to prepare hair fibers for cross-sectional microscopy.
Guidelines
Contamination is a risk:
- Use nitrile gloves when working with hair samples
- Tie hair back and/or wear hair net
- Wear lab coat if necessary (when fiber contamination risk, e.g. sweaters)
Materials
Embedding Materials
- Polly Plastic (polycaprolactone): Moldable Plastic Strips, 25 strips, 8" x 1" x 1/16" Thick
- Parchment paper
- Disposable Petri dishes
- Printer paper (white)
- Small coin envelopes
- Weigh boats
Microscope Imaging Materials
- Light microscope
- Camera w/ microscope attachment
- Computer monitor
- Camera HDMI Cord
- Embedded hair chips
- Plastic chip holder
Equipment
- 4C cold room/refrigerator
- Hot plate
- Weighted metal blocks (3 total; steel jewelers anvils work well)
- Small, straight scissors
- Metal tray for placing metal blocks
- Tweezers (2 total)
- PanaVise PanaPress with guillotine attachment or single blade
Troubleshooting
Preparing plastic chips for embedding
11m
The following steps are for cutting and thinning the (polycaprolactone) plastic to smaller size for embedding
Note
Polycaprolactone plastic melts at 60 °C . To produce small, thin chips for embedding, you will need:
- A sheet or strip of plastic cut into 2x2cm squares
- A hot plate (heated to 60 °C )
- 3 metal blocks to use as weights
- Scissors
- Squares of parchment paper (5x5cm)
- Heat resistant gloves
Safety information
Have adequate protection for your hands. The metal will be too hot to move with bare hands - use heat resistant gloves.
Heat plastic
- Heat one of the metal blocks on the hot plate for 5mins.
- Place square of plastic (with parchment paper underneath) on hot plate for 30 sec.
Flatten plastic.
- Cover heated plastic with square of parchment paper.
- Place heated metal block on top for 30 sec.
Cool plastic
- Remove plastic (with parchment on either side) and flatten between two cold metal blocks
- Leave to cool for 5 mins.
- Peel off parchment and remove thinned plastic chips.
Cut plastic to desired dimensions using scissors or scalpel.
Note
The appropriate size will depend on your hair samples. We recommend 20x10mm for most hairs and 20x5mm for very short hairs. Anything smaller will be challenging, though not impossible, to section.
Embedding Hairs
2h 6m
The following steps are for embedding hair fibers into the plastic chips.
Note
For embedding you will need:
- hair samples
- 2 prepared plastic chips per sample
- 2 tweezers/forceps
- a hot plate heated to 60 °C
- isopropanol for cleaning
Note
Prepare hair sample for embedding. Take 3-5 hairs per sample and cut samples down to 5cm if they are over this length.
Place one prepared plastic chip on a parchment paper on the heated block and wait until the chip is translucent (about 30sec).
Once plastic is translucent, grab a hair fiber with tweezers on each side and stretch over the plastic, pressing the hair into the plastic so it is completely submerged.
Continue until all hairs for that sample are embedded.
Note
Embedding the hairs
The hairs should be should be stretched taut over the plastic. In the process, some hairs may snap, so having spares is recommended.
Take care that all hairs are laid parallel to each other to ensure perpendicular cuts for the entire sample.
Heat second prepared plastic chip until translucent.
Carefully overlay the second chip over the first (containing the embedded hairs).
Apply metal block overtop to flatten sample for 15 sec.
Remove the embedded hair sample and place between two cool blocks and leave for 2mins.
When cooled, peel off parchment paper, trim excess hairs and plastic into a rectangular chip.
The sample should now be stored in a cool room at 4 °C for at least 2 hours.
Cutting Hairs
1m
Prior to cutting, the embedded chips must be cooled for at least 2hrs.
Note
Cutting the hairs will require:
- PanaVise with blade attachment (see comment)
- plastic bags for storing the embedded chips
Note
Cutting tools
Cutting the hair samples requires a sharp blade.
Our lab uses the PanaVise 502 Precision PanaPress with cable cutting attachment.
A sharp razor or other implement may work, but the blade must be sharp enough and it must be applied with enough pressure. Also, this must be done with minimal contact with the sample as body temperature will heat the embedded sample causing the hair to shift while being cut.
1m
Place chip under press and cut the sample in half. The sample may also be cut using a single blade.
Note
Work in small batches and work quickly as the chips will heat up.
You have the option of cutting the samples in half (resulting in two mirroring sections) or you can trim the top of a sample to expose the cross section.
Immediately store the samples in cold room or freezer until imaging.
Imaging
6m
For the imaging, you will require:
- a clamp or stand holding the sample upright (see materials)
- a microscope capable of magnifying to 10x
1m
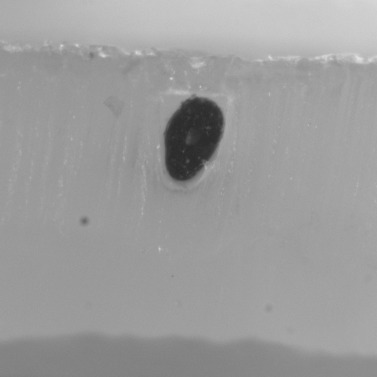
Mount the hairs vertically.
Image each hair in the embedded sample individually.
Note
Take care not to leave the samples under the microscope unattended, as the lights may melt the sample.
Store immediately in cool room or freezer.
