Jun 07, 2020
Protocol to immunostain Gastruloids (LSCB, EPFL)
- Stefano Vianello1,
- Mehmet Girgin2,
- Giuliana Rossi2,
- Matthias Lutolf3
- 1Marine Research Station, Institute of Cellular and Organismic Biology (ICOB), Avademia Sinica;
- 2Laboratory of Stem Cell Bioengineering, Institute of Bioengineering, School of Life Sciences (SV) and School of Engineering (STI), Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland;
- 3Laboratory of Stem Cell Bioengineering, Institute of Bioengineering, School of Life Sciences (SV) and School of Engineering (STI), Ecole Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, and Institute of Chemical Sciences and Engineering, School of Basic Science (SB), EPFL, Lausanne, Switzerland

Protocol Citation: Stefano Vianello, Mehmet Girgin, Giuliana Rossi, Matthias Lutolf 2020. Protocol to immunostain Gastruloids (LSCB, EPFL). protocols.io https://dx.doi.org/10.17504/protocols.io.7tzhnp6
License: This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Protocol status: Working
We use this protocol and it's working
Created: October 01, 2019
Last Modified: June 07, 2020
Protocol Integer ID: 28249
Keywords: Gastruloids, Immunstaining, Immunofluorescence, immunostaining gastruloid, mouse embryonic stem cell, lutolf lab, lscb, embryonic stem cell, epfl, gastruloid, published protocol, standard protocol, aggregates of mouse embryonic stem cell, protocol, cell
Abstract
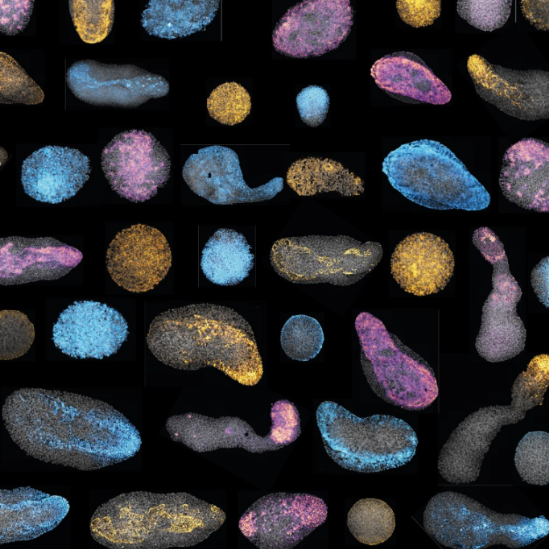
Standard protocol used for immunostaining Gastruloids (aggregates of mouse embryonic stem cells) in the Lutolf Lab, EPFL.
For a published protocol on how to generate Gastruloids, see:
Citation
LINK
Guidelines
All handling and transfer of Gastruloids from one solution to the next is done with a cut P1000 tip (to avoid damage). The tips should be coated in BSA- or serum-containing solution (in our case, the blocking PBS+FT solution itself). It is very important that all tips are coated, as Gastruloid will otherwise stick to the plastic and will be lost.
To transfer Gastruloids with minimal carry-over of liquid from one solution to the other, keep the pipette vertical until they all accumulate to the bottom of the tip. Slightly push to create a small hanging drop, and touch the surface of the new solution. All Gastruloids will transfer from the tip to the new solution. Hence, when the protocol calls e.g. for three PBS washes, three wells of a 6 well plate are filled with PBS, and Gastruloids moved serially from one to the other.
Materials
MATERIALS
DAPI Thermo Fisher ScientificCatalog #D1306
Triton X-100Merck MilliporeSigma (Sigma-Aldrich)Catalog #T8787
4% Paraformaldehyde in PBSAlfa AesarCatalog #J61899-AK
Permount mounting mediumFisher Scientific
Thermo Scientific™ SuperFrost Plus™ Adhesion slides Thermo Fisher ScientificCatalog #J1800AMNZ
PBS, pH 7.4Thermo FisherCatalog #10010015
Embryonic stem-cell FBS, qualified, US origin Thermo FisherCatalog #16141079
Fluoromount-GSouthern BiotechCatalog #0100-01
Greiner CELLSTAR® 6 well culture platesgreiner bio-oneCatalog #657185
Falcon™ 48well Polystyrene MicroplatesFalconCatalog #353078
#1 Micro Cover Glass 22mmx22mmElectron Microscopy SciencesCatalog #72200-10
The following recipes were used:
PBS-FT (PBS+FBS+ 0.2% Triton)
to make 500mL
- 450mL PBS, 1X [CAT#10010015, Gibco™/Thermo Fisher Scientific]
- 50mL ES-grade FBS [CAT#16141079, Gibco™/Thermo Fisher Scientific]
NOTE: could be replaced with BSA, less expensive
- 0.1mL Triton-X100 [CAT#T8787, Sigma Aldrich]
4% PFA in PBS (if not buying pre-prepared one):
NOTE: work under a hood, wearing a N95 mask, and appropriate protective equipment
to make 500mL
- Fill glass beaker with 400mL PBS, 1X [CAT#10010015, Gibco™/Thermo Fisher Scientific]
- Add magnetic stirrer
- Add 20g granulated PFA powder [CAT#0964.1, Carl Roth]
- Heat to 60°C (NOT HIGHER!) on a stirring hotplate, the solution will become transparent fom 55°C to 60°C
- When powder is dissolved, top up with PBS to 500mL total volume
- Adjust to pH7.4 with HCl or NaOH (5 drops 1MHCl)
Aliquot and store at -20°C medium- to long term
Troubleshooting
Safety warnings
This protocol involves the use of 4% Paraformaldehyde (4%PFA). Perform all steps involving 4%PFA under a chemical hood, and wear appropriate body and eye protection. Do not inhale vapours.
Before start
- Prepare PBS+FT (cfr. "Text Materials" for recipe)
- Coat wells of a 6-well plate with serum-containing buffer to prevent Gastruloids from sticking to it. Leave~30min at room temperature
- Bring 4%PFA to Room temperature
Fixing and primary antibody stain (D1)
Harvesting Gastruloids:
Using a cut P1000 tip to avoid accidental damage (cfr. "Guidelines"), collect Gastruloids from each well of the 96-well plate by placing the tip straight down to the bottom of each well, and aspirating up. Move to the next well until full tip capacity is reached.
Note
To increase reproducibility of analysis, do not collect Gastruloids from the outer wells of the plate (all 36 border wells). These tend to develop differently from all other Gastruloids, probably due to evaporation of the medium. We only collect Gastruloids from the inner 60 wells of the plate.
Deposit collected Gastruloids in a 15mL centrifuge tube as you go
When collected all Gastruloids needed, wait for them to sediment to the bottom of the tube, and remove as much medium as possible (vacuum pump+glass pipette)
Dilute away left-over traces of medium by gently resuspending the Gastruloids in ~5mL PBS-/-
Fixing (4%PFA):
Remove the serum-containing medium (in our case, PBSFT) from the 6-well plate (can keep and recycle to coat tips; cfr. "Guidelines") and replace with 2mL 4%PFA
Safety information
PFA is toxic. Operate under a fume hood and wear appropriate hand, body, and eye protection.
Collect all Gastruloids from the tube, wait for them to sink at the bottom of the tip, and touch the surface of the PFA vertically to transfer them with minimal liquid transfer (cfr. "Guidelines")
Safety information
Coat a cut P1000 tips in PBS+FT to avoi Gastruloids sticking to it!
Note
48h Gastruloids are particularly tricky to work with: they will stick and clump together, and they are almot invisible to the naked eye. To facilitate working with this timepoint, just push the whole contents of the tip into the next wash, without waiting for them to sink to the bottom of the tip, and without minding the carry over of medium from the previous wash. If possible, work with an excess of Gastruloids to compensate for loss during staining and washes.
Cover with aluminium foil to reduce fluorescence loss (when using reporter lines)
Incubate at 4 °C 02:00:00 , with or without orbital shaker
Washes (to remove PFA):
Fill wells of a 6-well plate with 4mL PBS-/- and transfer fixed Gastruloids using the same technique as above
Cover with aluminium foil and wait 00:10:00 min
repeat for two more washes
Note
If short on time, it is not necessary to wait 10 minutes for each wash. Gastruloids can be transferred serially across three PBS-filled wells, and left 10 minutes only in the last one.
PAUSE POINT: you can leave Gastruloids in the last PBS wash, for months, 4 °C , protected from light
Blocking:
Transfer Gastruloids to a well filled with 2mL PBS+FT
Block for 01:00:00 h, Room temperature , with or without shaker
Primary antibody staining:
For every antibody combination and sample condition you want to stain, prepare a 1.5mL Eppendorf tube with 500uL of the appropriate antibodies in PBSFT. Include DAPI 1:500.
Transfer each antibody solution in a separate well of a 48 well plate, and transfer equal number of Gastruloids to each well
Cover in aluminium and incubate 24:00:00 , 4 °C , on a orbital shaker
Secondary antibody stain (D2)
Washes (to remove 1ry Ab):
Wash Gastruloids by transferring them to a well of a 6-well plate filled with 3mL PBS+FT
Note
Previous versions of this protocol call for PBS-/- instead of PBS-FT. There is a much higher increase of Gastruloids still sticking to the bottom of the wells in this case however. PBS-FT seems to be the safest option.
Safety information
You will need a separate PBS-FT well for each different sample condition, do not mix all Gastruloids into the same well
Wait for 00:20:00 min
Repeat for two more washes (total: 01:00:00 h)
Note
Again, if short on time, it is not necessary to wait 20 minutes for each wash. Gastruloids can be transferred serially across three PBSFT-filled wells, and left 20 minutes only in the last one.
Secondary antibody staining:
For every antibody combination and sample condition stained, prepare 500uL of the appropriate secondary antibodies in PBSFT. Include DAPI 1:500 at this step too.
Transfer each antibody solution in a separate well of a 48 well plate, and transfer each Gastruloid set to its appropriate secondary solution
Cover in aluminium and incubate 24:00:00 , 4 °C , on a orbital shaker
Mounting (D3)
Washes (to remove 2ry Ab):
Wash Gastruloids by transferring them to a well of a 6-well plate filled with 3mL PBS+FT
Note
Previous versions of this protocol call for PBS-/- instead of PBS-FT. There is a much higher increase of Gastruloids still sticking to the bottom of the wells in this case however. PBS-FT seems to be the safest option.
Safety information
You will need a separate PBS-FT well for each different sample condition, do not mix all Gastruloids into the same well
Wait for 00:20:00 min
Repeat for two more washes (total: 01:00:00 h)
Note
Again, if short on time, it is not necessary to wait 20 minutes for each wash. Gastruloids can be transferred serially across three PBSFT-filled wells, and left 20 minutes only in the last one.
Mounting:
Take a microscope slide, and add a drop of ~30uL of Fluoromount-G (mounting medium) to the centre of it
Note
While we usually do not, a spacer can be used to separate the sample from the coverslip, and to preserve the exact morphology of the sample. The absece of a spacer is not particularly destructive for early Gastruloids, but some damage can be seen for 168h and later timepoints.
The spacer can be either bought commercially, or made with two layers of double-sided sticky tape. The drop of mounting medium would then be added within the area defined by the spacer.
Transfer Gastruloids to the drop (see technique in "Guidelines")
Note
You can transfer any number of Gastruloids to the same drop. Depending on the size of their size, this might however increase the chance that they cluster together (e.g. 144h, 168h). If this happens, you can try to separate them and distribute them more evenly with an eyelash tool, being careful not to damage them.
When big Gastruloids are used, it is generally recomended to do multiple slides with fewer Gastruloids, rather than one with many all clumping together.
Gently drop a coverslip on top of the samples
Note
To avoid trapping air bubble, deposit a small drop of mounting medium at the centre of the coverslip prior to use.
Using the edge of a Kimtech wipe, absorb out excess liquid seeping out of the coverslip edges
Seal all sides of the coverslip with Permount hardening resin (we filled an empty nailpolish bottle with Permount, and use the brush for handling)
Note
Nail polish can be used instead of Permount, but there are reports of alcohol possibly being able to seep into the mounting medium and reducing the long-term shelf-life of the sample.
Keep the slide in a box away from light, 4 °C , until ready for imaging
NOTE: slides can be kept at 4 °C for months before imaging with little apparent loss of signal. Try however to image slides as close as possible to when they were made
Citations
Beccari et al.. Generating Gastruloids from Mouse Embryonic Stem Cells
10.1038/protex.2018.094