May 01, 2025
Lipase-Catalyzed Acetylation of Racemic Citronellol and Determination of Enantioselectivity through Derivatization by: (i) Esterification or (ii) Oxidation-Hydrolysis
- Tu M. Ho1,
- Francis K. Yoshimoto1
- 1Department of Chemistry, The University of Texas at San Antonio (UTSA), One UTSA Circle, San Antonio, TX 78249
- Tu M. Ho: https://orcid.org/0009-0001-4625-8897
- Francis K. Yoshimoto: https://orcid.org/0000-0002-2308-2999

Protocol Citation: Tu M. Ho, Francis K. Yoshimoto 2025. Lipase-Catalyzed Acetylation of Racemic Citronellol and Determination of Enantioselectivity through Derivatization by: (i) Esterification or (ii) Oxidation-Hydrolysis. protocols.io https://dx.doi.org/10.17504/protocols.io.e6nvw83kdvmk/v1
License: This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Protocol status: Working
We use this protocol and it's working
Created: April 25, 2025
Last Modified: May 01, 2025
Protocol Integer ID: 164262
Keywords: Enzymatic kinetic resolution, Lipase acetylation, Citronellol, Vinyl acetate, Derivatization, Enantioselectivity, Liquid-liquid extraction, Biocatalysis, Oxidation, Aldehyde, Alcohol, Carboxylic acid, Dess Martin periodinane, Pinnick oxidation, Hydrolysis, catalyzed acetylation of racemic citronellol, enzymatic reaction, unreacted citronellol from the lipase reaction, catalyzed acetylation, protocol an enzymatic reaction, determination of enantioselectivity, mixture of enantiomer, lipase reaction, enantioselectivity, lipase from candida rugosa, biocatalysi, lipase product, citronellyl acetate, selective the enzyme, racemic mixture of citronellol, other during catalysis, topic of biocatalysi, racemic mixture of compound, catalysis, chiral molecules as substrate, esterification, acetylating reagent, lipase, racemic mixture of molecule, enzyme, chiral molecule, hydrolysis enzyme, enantiomer, citronellic acid, citronellol to citronellal, mixture from the pinnick reaction, racemic mixture, vinyl acetate, derivatization, racemic
Abstract
Enzymes have chiral active sites and, as a result, have the ability to recognize chiral molecules as substrates. With a racemic mixture of molecules (1 to 1 mixture of enantiomers), an enzyme should catalyze the reaction of one enantiomer over the other. In this protocol an enzymatic reaction was performed on a racemic mixture of compounds and the resulting mixture was derivatized to determine how selective the enzyme was in favoring one enantiomer over the other during catalysis. Specifically:
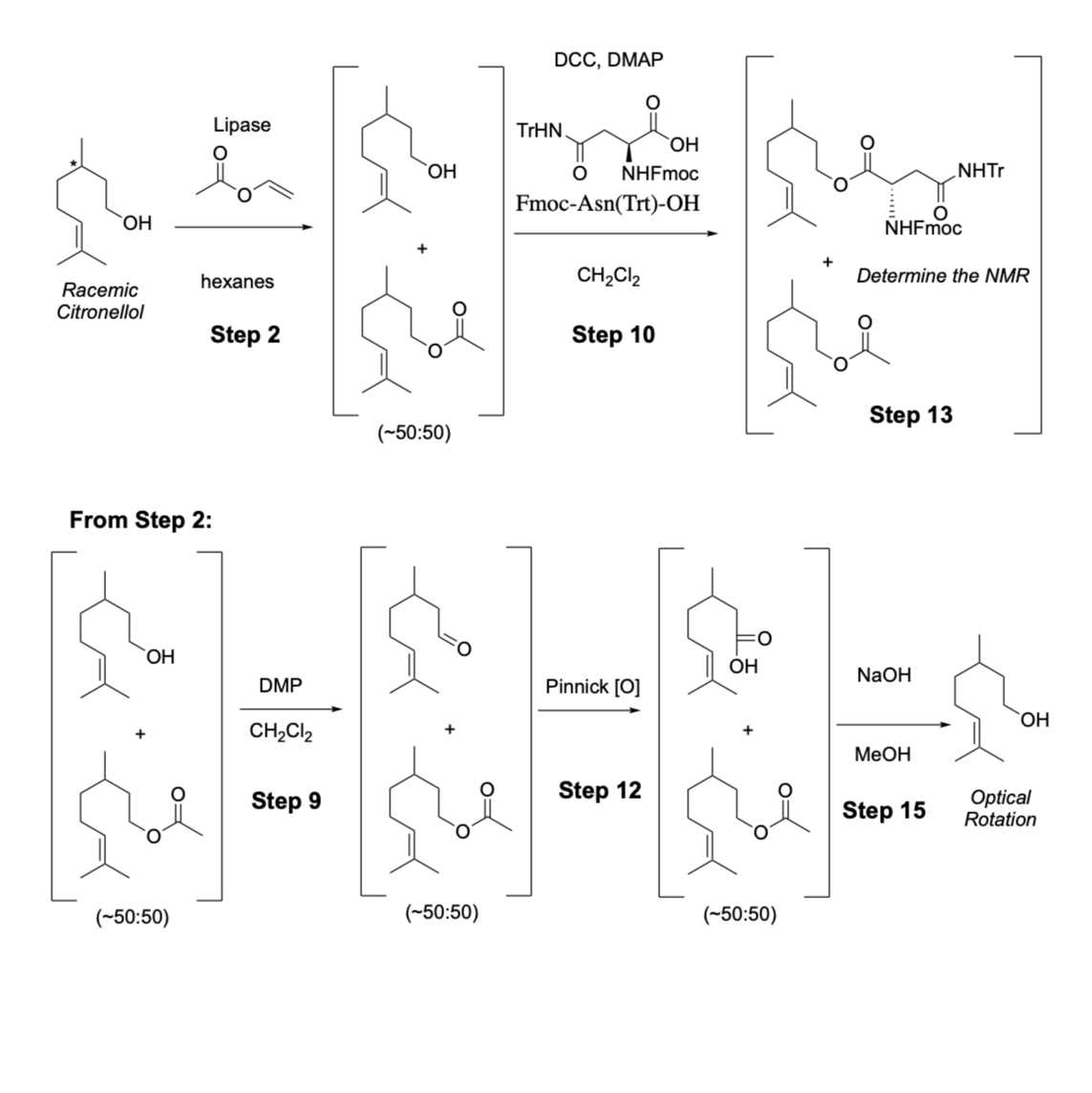
- A racemic mixture of citronellol was acetylated with lipase from Candida rugosa. Vinyl acetate was used as the acetylating reagent and hexanes was the solvent. The enzymatic reaction was monitored by thin layer chromatography and once the reaction reached 50% conversion, the reaction was terminated via filtration through celite.
- The unreacted citronellol from the lipase reaction was derivatized with either: (i) an asparagine derivative (Fmoc-Asn(Trt)-OH) to form an ester, or (ii) oxidized in two steps with Dess Martin periodinane followed by Pinnick oxidation to convert the citronellol to citronellal and citronellic acid, respectively. The resulting mixture from the Pinnick reaction was treated with basic conditions to yield citronellyl acetate (lipase product) in the organic layer while citronellic acid remained in the aqueous layer. The citronellyl acetate recovered from the Pinnick reaction was hydrolyzed to yield citronellol.
- The derivatization methods enabled the determination of enantioselectivity in the lipase reaction.
- This experimental protocol was used to teach the topic of biocatalysis in Spring 2025 at UTSA to a class of biochemistry students.
Troubleshooting
Safety warnings
Proper personal protective equipment must be worn at all times: safety goggles, nitrile gloves, lab coat.
Hexanes is flammable. CAM stain is corrosive.
Lipase-Catalyzed Acetylation of Racemic Citronellol and Determination of Enantioselectivity through Derivatization by: (i) Esterification or (ii) Oxidation-Hydrolysis
Gather materials required for the enzyme catalyzed reaction:
1. Safety goggles
2. Nitrile gloves
3. Lab coat
4. One 50-ml Falcon tube (Step 2) and four 15-ml Falcon tubes
5. Scale to weigh reagents (lipase powder in Step 2, Falcon tube in Step 16)
6. Lipase powder (Candida rugosa Type VII, L1754-25G, Sigma) (Step 2)
Lipase powder from Candida rugosa
8. citronellol (racemic) (CAS #: 106-22-9, MW: 156.27, d = 0.855) (Step 2)
9. vinyl acetate (CAS #: 108-05-4, MW: 86.09, d = 0.934) (Step 2)
10. water bath (35-40 °C)
11. TLC plates
12. Beaker (for TLC solvent chamber)
13. aluminum foil
14. Glass capillaries to spot TLC solutions (or Pasteur pipets or optionally, 1 μL pipet tips with 1-10 μL micropipettor)
15. CAM (ceric ammonium molybdate) stain https://www.protocols.io/view/extraction-of-food-items-and-analysis-with-thin-la-14egn3rdml5d/v1
16. Ethyl acetate (Step 4) https://www.fishersci.com/shop/products/ethyl-acetate-certified-acs-fisher-chemical-7/E1454
17. 10-ml disposable syringe with cotton plug then sand then celite to filter reactions
18. Cotton
19. Sand
20. Celite
21. apparatus to filter enzyme mixture (solid phase extraction)
22. house vacuum line
23. tubing to connect house vacuum line to the solid phase extraction apparatus
24. nitrogen evaporator carousel to connect to the house air line with tubing
25. Dess Martin periodinane (10% w/v in dichloromethane, CAS #: 87413-09-0, MW: 424.15) https://www.oakwoodchemical.com/ProductsList.aspx?CategoryID=-2&txtSearch=195363&ExtHyperLink=1
26. dichloromethane (Step 8, Step 10) https://www.fishersci.com/shop/products/methylene-chloride-stabilized-certified-acs-fisher-chemical-7/D37500
27. Nα-(9-Fluorenylmethoxycarbonyl)-Nγ-trityl-L-asparagine, Nα-Fmoc-Nγ-trityl-L-asparagine or Fmoc-Asn(Trt)-OH (CAS #: 132388-59-1, MW: 596.67) https://www.sigmaaldrich.com/US/en/product/aldrich/47672?srsltid=AfmBOoqj95zoNe6SZhNYeSpLkJaxiVHk_Nu2GrJq19_2bo_0vGk498R_ (Step 10)
33. tert-butanol (Step 11) https://www.fishersci.com/shop/products/tert-butanol-certified-fisher-chemical-2/A4011#?keyword=
34. Pasteur pipet (Step 14)
35. Pipet bulb (Step 14)
36. Methanol (Step 15)
37. NaOH in water (30% w./v, 3 g NaOH in 10 ml water) (Step 15)
38. Sharpee for labeling
39. heat gun or hot plate to visualize spots on the TLC plate after CAM stain (Step 17)
40. Plastic tube rack https://www.fishersci.com/us/en/browse/90086063/tube-racks?categoryKey=90086063&page=2
Set up the lipase reaction:
In a 50-mL falcon tube, weigh out 200 mg of lipase powder, then add 10 mL of hexanes follow by citronellol (120 μL, 0.103 g, 0.657 mmol, 1.0 equiv) and vinyl acetate (250 μL, 0.234 g, 2.71 mmol, 4.1 equiv).
Lipase reaction set up. Left image: weighing lipase reagent into 50-mL Falcon tube. Right image: placing reagents into the Falcon tube and into the water bath.
Start the lipase reaction (lipase-catalyzed acetylation of citronellol):
Incubate the reaction in water bath at 35 – 40 °C in 30 minutes.
Note: Reaction should be monitored by TLC to make sure 50% conversion.
Preparation of the TLC chamber:
While waiting for the reaction from step 3, prepare the standard starting material for the TLC: add 25 μL of citronellol to 1 mL of ethyl acetate. Prepare the TLC solvent (10 mL, 4:1 hexanes/ ethyl acetate) and TLC plate with 7 lanes as follow:
- Lane 1: citronellol (racemic)
- Lane 2: lipase reaction mixture after celite filtration (Step 5)
- Lane 3: Esterified unreacted citronellol with the asparagine derivative (RXN B, Step 13)
- Lane 4: DMP reaction (RXN A1, Step 9)
- Lane 5: Oxidized aldehyde/acetate mixture from Pinnick Reaction (RXN A2, Step 14), organic layer
- Lane 6: Oxidized aldehyde/acetate mixture from Pinnick Reaction (RXN A2, Step 14), aqueous layer
- Lane 7: Hydrolyzed citronellyl acetate (Step 15)
Working up the reaction from Step 3 through filtration:
After 30 minutes - the TLC analysis confirmed the 50% conversion. Remove the reaction from the water bath and filter the lipase residue through a syringe packed with cotton pad, sand and celite. Spot the solution on lane 2 of the TLC plate in Step 4.
*In the video below, the reaction from Step 3 (lipase powder, vinyl acetate, citronellol, hexanes) is poured through a disposable syringe fitted with a cotton plug, sand, and celite to filter out the lipase powder. The syringe was placed on a solid phase extraction apparatus connected to the house vacuum with tubing. The clear solution that is collected in the 15-ml Falcon tube is carried forward to Step 6.
Setup to filter off lipase powder described in Step 5: Solid phase extraction apparatus connected to the vacuum line and fitted with a 15-mL Falcon tube and above the tube is the 10-mL plastic syringe with a cotton plug and sand and celite.
Filter the enzyme reaction mixture through celite.
Split the filtrate from Step 5 (about 9 mL) into 2 separate 15-ml falcon tubes:
- RXN A: about 6 mL
- RXN B: about 3 mL
Filtrate from Step 5 came out clear with no solid indicating successful removal of lipase powder. The main solution is split into two 15-mL Falcon tubes: RXN A (6 mL) and RXN B (3 mL).
Concentration of RXN B from Step 6:
Evaporate hexanes solvent in RXN B from Step 6 through air flow (should take about 15 minutes) in the carousel.
Oxidization of RXN A from Step 6 with DMP (RXN A1):
To RXN A, add 1.7 mL of Dess-Martin Periodinane solution (10% w/v in DCM) and wait 30 minutes at room temperature. Prior to measuring out the DMP solution and adding to the mixture, make sure that the stock solution of DMP (10 wt% w/v) is shaken thoroughly since the white precipitate (reagent) settles on the bottom of the solution. The reaction should be shaken and mixed every 5 minutes.
Oxidation of the citronellol to citronellal with DMP. Set up Dess-Martin Periodinane reaction (Step 8, RXN A).
Filtration of the DMP reaction from Step 8 (RXN A1):
After 30 minutes, filter RXN A (DMP reaction) from Step 8 through a syringe column (similar to Step 5). Use minimal amount of DCM to rinse the residue in the tube (about 2 mL). Label the collected filtrate as “RXN A1” and spot the solution the solution on lane 4 of TLC plate from Step 4. The filtrate is then evaporated under air until mostly no liquid is presented. DMP converts the alcohol (citronellol) to an aldehyde (citronellal).
Left image: DMP reaction after 30 minutes from Step 8. Right image: Filtration of the DMP reaction (Step 9, RXN A).
Esterification of the unreacted citronellol from Step 7 with asparagine derivative (RXN B):
To RXN B from Step 7, set up the Asparagine derivatization reaction: add DCM (4 mL), DMAP (8 mg, 0.033 mmol), DCC (66 mg, 0.32 mmol), and Fmoc-Asn(Trt)-OH (190 mg, 0.32 mmol) reagent. The solution should turn yellow once Fmoc-Asn(Trt)-OH is added. The reaction is run at room temperature (shake and mix reaction occasionally) (continue to Step 13).
Esterification of the reaction with the Asparagine derivative (Step 10, RXN B)
Preparation of the Pinnick oxidation solution for RXN A2 (the solution will be used in Step 12):
While waiting for the solvent in RXN A1 to evaporate (Step 9), prepare the solution to set up the Pinnick reaction:
11.1. NaClO2 solution: weigh out 200 mg NaClO2 (2.2 mmol) and dissolve in 400 μL H2O. (Step 11.1)
11.2. Solution X: 2 mL of tBuOH, 500 μL of H2O, 450 μL of 2-methyl-2-butene, 192 mg of NaH2PO4. (Step 11.2)
Oxidation of the aldehyde/acetate mixture from Step 9 under Pinnick conditions (RXN A2):
To RXN A1 tube (after most of the solvent is evaporated), add 200 μL of NaClO2 solution (Step 11.1) and 2 mL of solution X (Step 11.2). The reaction is run at room temperature for 30 minutes (shake and mix the reaction occasionally by hand). The Pinnick reaction converts the aldehyde to the carboxylic acid (citronellal to citronellic acid). Continue to Step 14.
Set up Pinnick reaction (Step 12).
NMR sample preparation of the esterified unreacted citronellol with asparagine derivative from Step 10 (RXN B):
RXN B: After 30 minutes of the reaction involving derivatization with the asparagine derivative and DCC (Step 10), add 5 mL of water then shake and vent (x3). Use pipet to draw out the organic layer (bottom layer) and transfer to a clean 15-mL falcon tube labeled “Organic B”. Spot the solution on lane 3 of the TLC in Step 4. Evaporate “organic B” layer under air flow until no solvent is left, then take the NMR of the crude by adding 500 μL of CDCl3.
Extraction of esterification reaction with water from Step 10. Two layers were formed (top layer: aqueous, bottom layer: organic comprised of DCM) and yellow color faded after 10 minutes at room temperature (Step 13).
Separation of citronellic acid from citronellyl acetate in RXN A2 (continued from Step 12):
RXN A1: After 30 minutes of the Pinnick oxidation (Step 12), add 2 mL of NaOH 30% (w/v) solution (pH 11) and 2 mL of hexanes. Shake and vent the mixture, then separate the bottom aqueous layer using a pipet. Repeat the extraction two more times (2 x 2 mL NaOH 30%) to remove all of the citronellic acid (in the aqueous layer, bottom layer). Combine 3 aqueous layers in a 15-mL falcon tube labeled “aqueous A2” and spot the solution on lane 6 of the TLC in Step 4. The organic layer (top layer comprised of hexanes) is left and spotted on lane 5 of the TLC in Step 4.
Hydrolysis of citronellyl acetate from Step 14 (RXN A3):
To the organic layer (top layer) in Step 14, add 2 mL NaOH 30% (w/v) solution and 2 mL of MeOH. Shake the reaction vigorously for at least 5 minutes until all citronellyl acetate is hydrolyzed. The reaction should take about 30 minutes to complete and constantly shaking helps the reaction to complete faster.
Optical rotation of the citronellol recovered from Step 15:
After 30 minutes, separate the organic top layer from Step 15 using a pipet, then add 2 mL of hexanes to the aqueous layer and repeat to extract the top layer again. Spot the organic (top) layer on lane 7 of the TLC plate from Step 4. The organic layer is evaporated in a tared 15-mL Falcon tube to give citronellol (the citronellol that was converted to citronellyl acetate by lipase in Steps 2-3, then hydrolyzed in Step 15). Once the organic solvent is evaporated completely, mark the weight to determine how much citronellol is in the tube. The optical rotation is taken by adding 10 mL of DCM to 5-10 μL of citronellol.
TLC analysis:
After all reactions are spotted on the TLC, place it in the TLC chamber prepared in Step 4. When placing the TLC plate in the chamber, ensure that the solvent level in the TLC chamber is below the spots of origin on the TLC plate (otherwise the spots will not develop). Once the solvent is developed, dry the solvent and stain the TLC plate with CAM stain solution. Dry excess stain on the TLC plate with a paper towel, then apply gentle heat (with a hot plate or heat gun) until all spots appear. Calculate Rf values of each "spot" (the distance traveled by the spot from the origin divided by the distance from the solvent front to the origin).
From left to right:
Lane 1: Citronellol (racemic) (Step 1)
Lane 2: Lipase reaction (Step 5)
Lane 3: Esterified unreacted citronellol with asparagine derivative (RXN B, Step 13)
Lane 4: DMP reaction (RXN A1, Step 9)
Lane 5: Pinnick reaction (Oxidized aldehyde/acetate mixture) (RXN A2, Step 14), organic layer
Lane 6: Pinnick reaction (Oxidized aldehyde/acetate mixture) (RXN A2, Step 14), aqueous layer
Lane 7: Hydrolyzed citronellyl acetate (Step 15)
NMR analysis:
In order to facilitate the understanding of the new derivatization technique to determine the enantiomeric excess, individual citronellol stereoisomers (specifically: S-citronellol, racemic citronellol, ) were esterified with the Fmoc-Tr-Asn-OH derivative and the NMR spectra were obtained and uploaded on NMRXIV: https://nmrxiv.org/P110
1. The asparagine reagent (Fmoc-Trt-Asn-OH) https://nmrxiv.org/S1136
2. Derivatized racemic citronellol https://nmrxiv.org/S1137
3. Derivatized S-citronellol https://nmrxiv.org/S1135
4. Derivatized unreacted citronellol (from the lipase reaction) https://nmrxiv.org/S1138
5. Derivatized hydrolyzed citronellyl acetate (from the lipase reaction) https://nmrxiv.org/S1139
