Oct 20, 2020
Implantation of a pelvic nerve array in rats_original from SP
- 1Bionics Institute;
- 2University of Melbourne
- SPARCTech. support email: info@neuinfo.org

Protocol Citation: James B Fallon, Sophie Payne, Janet R Keast, Peregrine B Osborne 2020. Implantation of a pelvic nerve array in rats_original from SP. protocols.io https://dx.doi.org/10.17504/protocols.io.bfwxjpfn
License: This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Protocol status: Working
Created: May 03, 2020
Last Modified: November 26, 2023
Protocol Integer ID: 36535
Keywords: Peripheral nerve stimulation
Abstract
A pelvic nerve array is custom designed for implantation onto the pelvic nerve of male rats. This device is used for long-term (recovery) experiments. The device can electrically stimulate the pelvic nerve as well as record electrically evoked or spontaneous neural activity. Previously, we have successfully implanted rats for 2 months with no reports of biological adverse complications or damage sustained to the array. The surgical procedure is performed under anesthesia and should incorporate all local requirements for standards of animal experimentation, including methods of anesthesia, surgical environment, and post-operative monitoring and care.
Materials
MATERIALS
CarprofenZoetis
IsofluraneZoetisCatalog #10015516
Hartman’s SolutionProVet
BetadineZoetis
Pelvic nerve electrode arrayBionics Institute
LacrilubeEllar Laboratories
Fine Forceps - Mirror FinishFine Science ToolsCatalog #11412-11
Micro-Adson forcepsFine Science ToolsCatalog #11018-12
Halsey Micro needle holderFine Science ToolsCatalog #12500-12
Fine scissors - SharpFine Science ToolsCatalog #14060-09
Surgical Preparation
Surgical Preparation
Prior to surgery, pelvic nerve arrays are sterilised through a sonication process and autoclaving.
Sonication cycles: 15 mins in 5% Pyroneg, 5 mins distilled water, 5 mins in distilled water, 10 mins in 96% ethanol, 5 mins in distilled water, 5 mins in distilled water
Autoclave
For surgery, anesthetise (2.5% isoflurane in oxygen, or as required for maintenance) the animal (adult male rat Sprague-Dawley, 8-10 weeks)
Apply eye lubricant and place animal on a heat pad
Shave and clean (betadine) the ventral abdomen and the dorsal-lumbar back
Surgery
Surgery
Make an incision to the dorsal-lumbar aspect of the back (this will be the exit site for the percutaneous electrode connector).
Make a ventral midline incision in the skin overlying the region of the bladder
Using hemostats, subcutaneously tunnel the array from the dorsal incision site through to the ventral incision site.
Make an incision in the ventral midline of the abdominal cavity to expose the bladder.
Make a second, small (1 cm) incision (lateral to the midline incision) to the abdominal muscle on the same side as lead wire, and feed the array through this secondary incision.
Locate the left or the right prostate and gently retract the lobe to reveal the pelvic ganglia and connecting nerves (including the pelvic nerve)
Clearly identify the pelvic nerve
The array is placed over the top of the pelvic nerve and secured in place to the connective tissue of the prostate using sutures (7’0 silk).
Exteriorise the lead wire through the abdominal cavity (via the second small incision).
At this stage of the surgery, a bladder catheter can be implanted according to the protocol described in 'Cystometry for Awake Rats'
Protocol
NAME
Cystometry in awake rats
CREATED BY
Janet R Keast
Prepare bladder cannula at least 24 - 48 h prior to surgery. This is comprised of a short length of PE tubing that connects directly with the bladder lumen; this tubing inserts into a long piece of PVC tubing that is exteriorised at the base of the neck.
Note
The length of PVC tubing required is ~25 cm (male rat) and ~20 cm (female rat). This length includes room for the animal to grow and to provide sufficient amount exposed at the neck for daily flushing and experimental cystometry. The length of PE tubing required is ~ 2 cm.
- By carefully holding PE tubing over an open flame, flare the end to form a bell shape. Ensure that the lumen of the tubing does not seal during this process.
- Cut the flared end to a length of 0.5-1 cm.
- Insert the non-flared end into the end of the PVC tubing as far east it will go (usually 2-3 mm)
- Secure by applying superglue to the joint.
- To make the collar (securing point used for attaching to the neck skin): cut 0.5-1 cm of silastic tubing and glue it to the exteriorised end of the cannula tubing (leaving 5 cm overhang).
Anesthetise animal (2.5% isoflurane in oxygen, or as required for maintenance)
Shave and clean the ventral abdomen and the dorsal neck.
Apply eye lubricant and place animal on heated pad.
Perform a ventral midline incision in the skin overlying the region of the bladder. Place gauze dampened with sterile 0.9% saline over the abdominal incision site and turn the animal over.
Perform a dorsal midline incision in the skin at the base of the neck. This will be the exit site for the bladder cannula.
Blunt-dissect under the skin from this neck incision site to the ventral abdominal incision side using hemostats. Gently pull the bladder cannula tubing through.
Perform a ventral midline incision in the muscle. Make a smaller secondary incision in the abdominal muscle on the same side as the cannula tubing being tunnelled. Feed the cannula through the hole to enter the abdominal region.
Using cotton-tipped applicators dampened with sterile 0.9% saline, gently expose the bladder. Using 4’0 PDS suture, place a very loose purse string knot around the tip of the bladder dome. This should be located between a quarter and a third of the way down the apex of the dome. DO NOT TIGHTEN.
Pierce the tip of the bladder dome with a 19G needle, carefully absorbing any leaked urine with gauze. Use fine forceps to widen the hole into the bladder.
Fill the cannula with 200-300 µl sterile saline and insert flared end of the cannula into the bladder, ensuring that the end terminates below the location of the purse string suture. Close the purse string suture and tie off.
Once secured, infuse 0.2 ml 0.9% sterile saline into the bladder to check for leaks. If there are leaks, re-tie slightly lower than the original suture.
Close the muscle wall and abdominal skin using approved procedures.
At the dorsal neck, orientate the collar and cannula tubing so they face caudally. Place a suture through the neck skin, then collar, and then opposite side of the neck skin, then tighten firmly. Repeat this step two more times to secure the collar to the skin.
Close the end of the exteriorised cannula using a blunt 25G needle that has been sealed at one end with superglue.
Administer analgesics and monitor animal during postoperative period as per local approved procedures. To maintain patency of the cannula, infuse with gentamicin (0.2 ml, 40 mg/ml in sterile 0.9% saline) for 3 d and then daily with sterile 0.9% saline (0.5 ml).
After post-surgical recovery, habituate the animal to the recording chamber (30 min per day) at least 3 days before cystometry recording
Place the animal into the experiment chamber. Fill up a 25 ml syringe with sterile 0.9% saline. Fill the transducer and connection to the bladder cannula with sterile 0.9% saline.
Set the baseline pressure (Labchart) by holding a flowing tube of saline at the transducer level prior to inserting it into the cannula line of the rat. Allow this to run for a minute before connecting the line to the animal.
Slowly infuse sterile 0.9% saline into the bladder at rate 0.1 ml/min or the rate required for your experiment
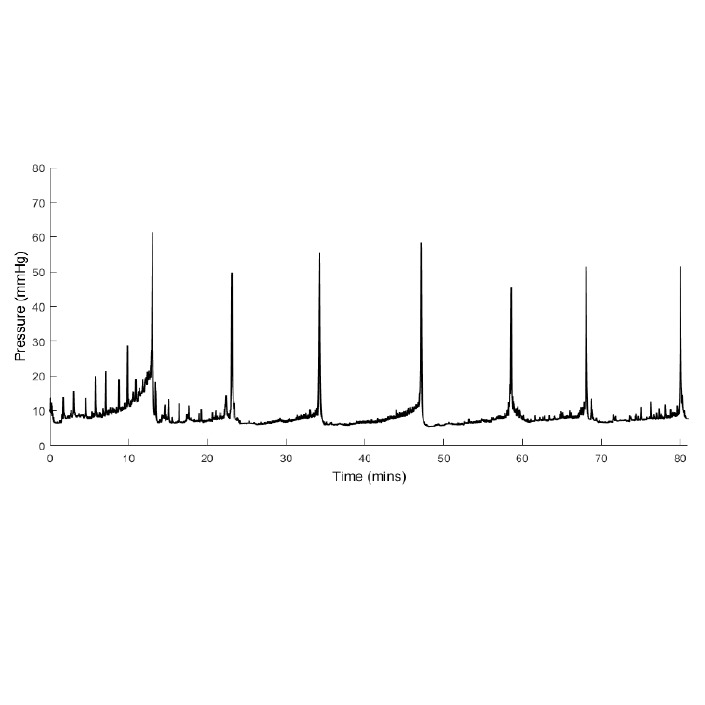
After inserting the line into the cannula, record bladder pressure for 30 min while the rat settles. Continue cystometry for approximately another 90 min or the duration required for the experiment.
Switch off the pump, disconnect the cannula from the pump and return the animal to its home cage for 2 hours.
To analyse Immediate early gene activation in spinal cord, 2 hours after finishing the cystometry recording, anesthetise and fix animals by intra-cardiac perfusion, then remove spinal cord for further study. This 2 hour period is required for expression of immediate early genes.
Close the abdominal cavity and skin incision sites with sutures.
Suture (2’0 silk) the percutaneous pedestal silicone-Dacron embedded mesh to the underlying muscle and connective tissue of the dorsal midline near the neck.
Close the skin incision site with sutures.
Administer analgesic and sterile Hartmann’s solution.
During post-surgery management, the skin around the percutaneous plug is cleaned with betadine every 2 weeks to ensure an infection does not develop. Rats can be awake, but gently restrained, during this maintenance procedure.
