Apr 25, 2025
Version 2
Crystallisation of Enterovirus D68 3C protease in space group P21 used for follow up compounds V.2
- Ryan Lithgo1,2,
- Peter Marples1,3,2,
- Lizbé Koekemoer4,2,
- Daren Fearon1,3,2
- 1Diamond Light Source;
- 2ASAP Discovery Consortium;
- 3Research Complex at Harwell;
- 4Centre of Medicines Discovery, University of Oxford
- Ryan Lithgo: The principle crystallographer on the Enterovirus 3C protease project.;
- ASAP Discovery

Protocol Citation: Ryan Lithgo, Peter Marples, Lizbé Koekemoer, Daren Fearon 2025. Crystallisation of Enterovirus D68 3C protease in space group P21 used for follow up compounds. protocols.io https://dx.doi.org/10.17504/protocols.io.5qpvoky29l4o/v2Version created by Mary-Ann Xavier
License: This is an open access protocol distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Protocol status: Working
We use this protocol and it's working
Created: November 12, 2024
Last Modified: April 25, 2025
Protocol Integer ID: 111973
Keywords: crystallisation, 3C protease, XChem, ASAP, AViDD, CMD, Diamond Light Source, i04-1, D68 3C protease, crystallisation of enterovirus d68 3c protease, enterovirus d68 3c protease, emerging pathogen enterovirus d68, pathogen enterovirus d68, potential target for antiviral drug development, d68 3c crystal, antiviral drug development, 3c protease of ev, spectrum antiviral, essential role in the viral life cycle, screening crystallography, 3c protease, viral life cycle, protocols for protein expression, protein expression, pdb group deposition g-10002271, space group p21, crystallisation, d68, protein
Funders Acknowledgements:
National Institutes of Health/National Institute Of Allergy and Infectious Diseases (NIH/NIAID)
Grant ID: Grant ID: U19AI171399
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgements:
Diamond Light Source Ltd, Harwell Science and Innovation Campus, Didcot OX11 0QX, UK
Research Complex at Harwell, Harwell Science and Innovation Campus, Didcot OX11 0FA, UK
Oxford Lab Technologies crystal shifter https://doi.org/10.1107/S2059798320014114
Abstract
The development of effective broad-spectrum antivirals forms an important part of preparing for future pandemics. A current cause for concern is the emerging pathogen Enterovirus D68 (EV-D68), which primarily spreads through respiratory routes. While it mostly causes mild to severe respiratory illness, in severe cases it can lead to acute flaccid myelitis. The 3C protease of EV-D68 is a potential target for antiviral drug development due to its essential role in the viral life cycle and high sequence conservation. This protocol was used to grow EV-D68 3C crystals that were subjected to high-throughput fragment screening crystallography (PDB group deposition G_10002271). In this new version, we have added the protocols for protein expression and purification, soaking conditions, and fragment screening information, as well as the affiliation with the ASAP Discovery Consortium.
Materials
SwissCI 3 lens crystallization plates https://swissci.com/product/3-lens-crystallisation-plate/ Codes:
Midi: UVXPO-3LENS 3W96T-PS 3W96T-UVP
1 Molarity (M) Tris adjusted to 7.8 with NaOH, Molecular Dimensions, Catalog # MD2-027-PH 7.8
1 Molarity (M) Ammonium acetate, Molecular Dimensions, Catalog # MD2-002-PH
50% w/v PEG 3350, Molecular Dimensions, Catalog # MD2-250-9
Purified D683C protein (35 mg/mL ) in 10 millimolar (mM) HEPES, 7.5 , 0.5 Molarity (M) NaCl, 5% glycerol, 0.5 millimolar (mM) TCEP
Protocol materials
Enterovirus D68 Strain STL 2010 12 3C proteaseaddgeneCatalog #228644
Troubleshooting
Safety warnings
Follow all handling warning for the chemicals used in the crystalllisation screen composition.
Protein expression and purification protocol
The protein used for crystallography used the following protocol for expression and purification.Enterovirus D68 Strain STL 2010 12 3C proteaseaddgeneCatalog #228644
Protocol
CREATED BY
Korvus Wang
Equipment needed
Crystallization experiment
1d
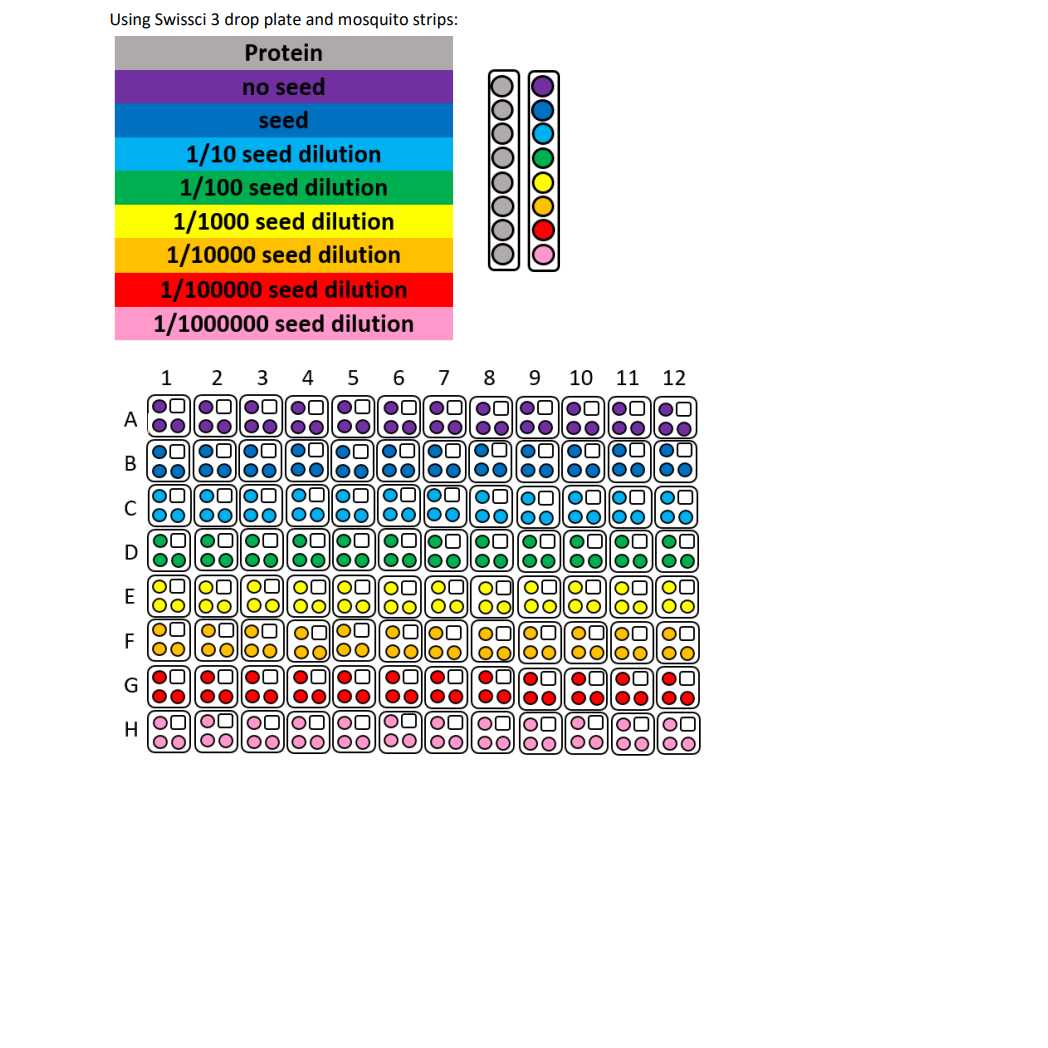
1: 1 000 000 dilution Sample seeds
Protein and buffer requirements:
14.4 µL 35 mg/mL Sample
2.88 mL Crystallization screen
7.2 µL seeds, dilution 1:1 000 000
Crystallisation screen composition:
0.1 Molarity (M) Tris 7.8
0.2 Molarity (M) Ammonium acetate
26% w/v PEG 3350
Stock solutions used:
1 Molarity (M) Tris adjusted to 7.8 with NaOH
1 Molarity (M) Ammonium acetate
50% w/v PEG 3350
Note
The crystallisation screen can be stored in a duran bottle or aliquoted into 96 deep well block for easy dispensing into SwissCI 3 lens plates.
For long term storage keep the Crystallisation screen in the fridge at 4°C.
Dispense 30 µL Crystallisation screen into SwissCI 3 lens plate reservoir wells using a 100 µl multi-channel pipette.
Dispense 50 µL 35 mg/mL Sample to each lens using the SPT mosquito.
Dispense 100 µL Crystallisation screen to each lens using the SPT mosquito.
Dispense 25 µL Seeds to each lens using the SPT mosquito.
Drop ratio: 2:4:1
Final drop volume: 175 nl
Incubate at 20 °C for 24:00:00 h in Formulatrix Rock Imager.
Imaging Schedule: The first images are taken after 12 h and the imaging schedule follows a Fibonacci sequence of days for further collections.
1d
Expected result
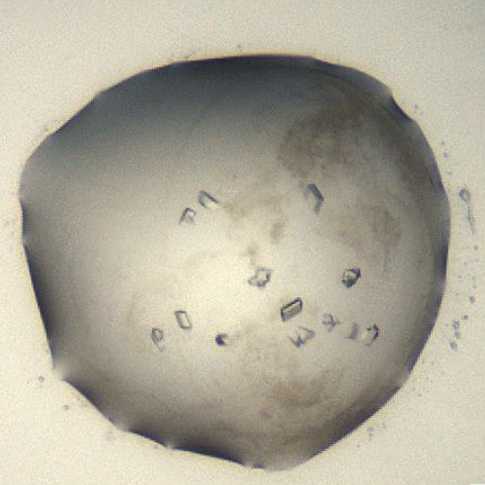
EEV-Crystals typically appear after 24 hours and reach their maximum size after ~24 h with some precipitation often remaining.
Morphology: small shards.
Size: ~40 μm in length and ~40 μm in width, depth of the crystals is ~20 μm, giving a
glass shard appearance
Average resolution: 1.5 Å
Space group: P21
Unit cell: 39.7 Å, 105 Å, 43.5 Å
90.00°, 110.00°, 90.00°
An example of a drop containing EV-D68 3C protease crystals.
Data collection at Synchrotron
Diamond Light Source
Unattended Data Collection (UDC)
Data Collection Temperature: 100K
Detector: DECTRIS EIGER2 X 9M
Beamline: I04-1
Wavelength: 0.9212 Å
Resolution (Å): 1.62
Beam Size (μm): 60 X 50
Number of images: 3600
Oscillation: 0.10°
Exposure (s): 0.0020
Transmission (%): 100
Flux (ph/s): 9.50e+11
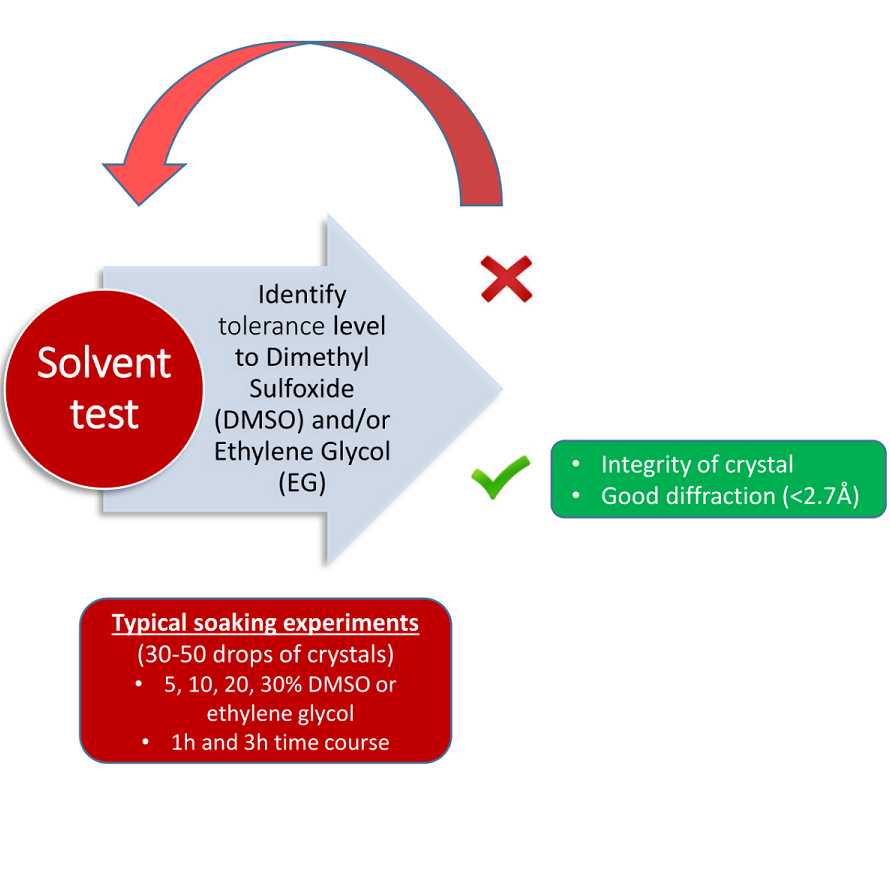
Soaking Tolerance
2h 30m
Final condition idenitified: 20% DMSO 02:30:00
2h 30m
Compound Soaking
2h 30m
Condition used: 20% compound, 02:30:00
2h 30m